Data Integrity and Life Sciences Manufacturers
Manufacturers are constantly looking for innovative ways to use technology to extract, process, and visualise data for important business insights. These insights created by data integrity provide workers and organizations with decisions, maximize operational efficiencies and productivities. Moreover, detailed and accurate production data is important for showing compliance throughout periodic audits, particularly in pharmaceutical and medical device manufacturing.
To guarantee data integrity, regulatory bodies have created guidelines for manufacturers. Precisely, the United States Food and Drug Administration (FDA) presented the ALCOA principles to guide data integrity and collection within the life sciences industry.
In this article, we will explore ALCOA principles, how life sciences manufacturers can apply them to their operations and their significance.
What is ALCOA for Data Integrity?
ALCOA is a set of principles which are decided by the FDA to keep track of data integrity in manufacturing, produced as guidance under 21 CFR Part 11 in 2018. Life sciences manufacturers use these guidelines to make sure products are made safely following all approved methods.
The full form of ALCOA is attributable, legible, contemporaneous, original, and accurate. By including these principles in processes, businesses can make sure the data collected is accurate and the organisation follows the defined methods.
As manufacturing processes and technologies grow, regulators have increased ALCOA principles to include complete, enduring, consistent, and available. These are collectively referred to as ALCOA+.
The 9 Principles of ALCOA
There are nine principles which every manufacturing organisation must follow as per FDA. These principles are:
Attributable |
Legible |
Contemporaneous |
Original |
Accurate |
Complete |
Consistent |
Enduring |
Available |
Attributable
This principle provides a clear picture of the data as it helps in recognising and recording the body responsible for data acquisition, including the time and date of collection.
Legible
This principle in comparison to paper-based documents uses digital data extraction and recording to minimise errors and improve readability.
Contemporaneous
This one records the accurate time of data collection and any subsequent alterations.
Original
Here it utilises original data in the main record while relying on primary data for further processing.
Accurate
Accurate principal makes sure that unedited and accurate data in input for reliable analysis and business decisions.
Complete
It maintains all created data with a comprehensive audit trail displaying alterations and their timings.
Consistent
This principle makes sure that all data records are reliable, and alterations are sequentially traceable.
Enduring
This principle keeps the records for a long time as important data might be required years or decades later using local and cloud dependent backups.
Available
This principle makes sure that data is accessible to authorised bodies whenever required.
These were the principals, let us now move forward to the importance of data integrity.
The Importance of Data Integrity in Life Sciences Manufacturing
Life sciences manufacturers face strict scrutiny because of the possible impact on consumer well-being. Periodic audits by regulators just like the requirements of FDA to ALCOA+ principles are compulsory to maintain all GMP compliance.
For organisations decisions and constant improvements, accurate and easily accessible production data is important. Transparent data and information guarantee traceability, identification of inefficiencies and safety. This enables the organisation to have corrective actions without any delays or issues.
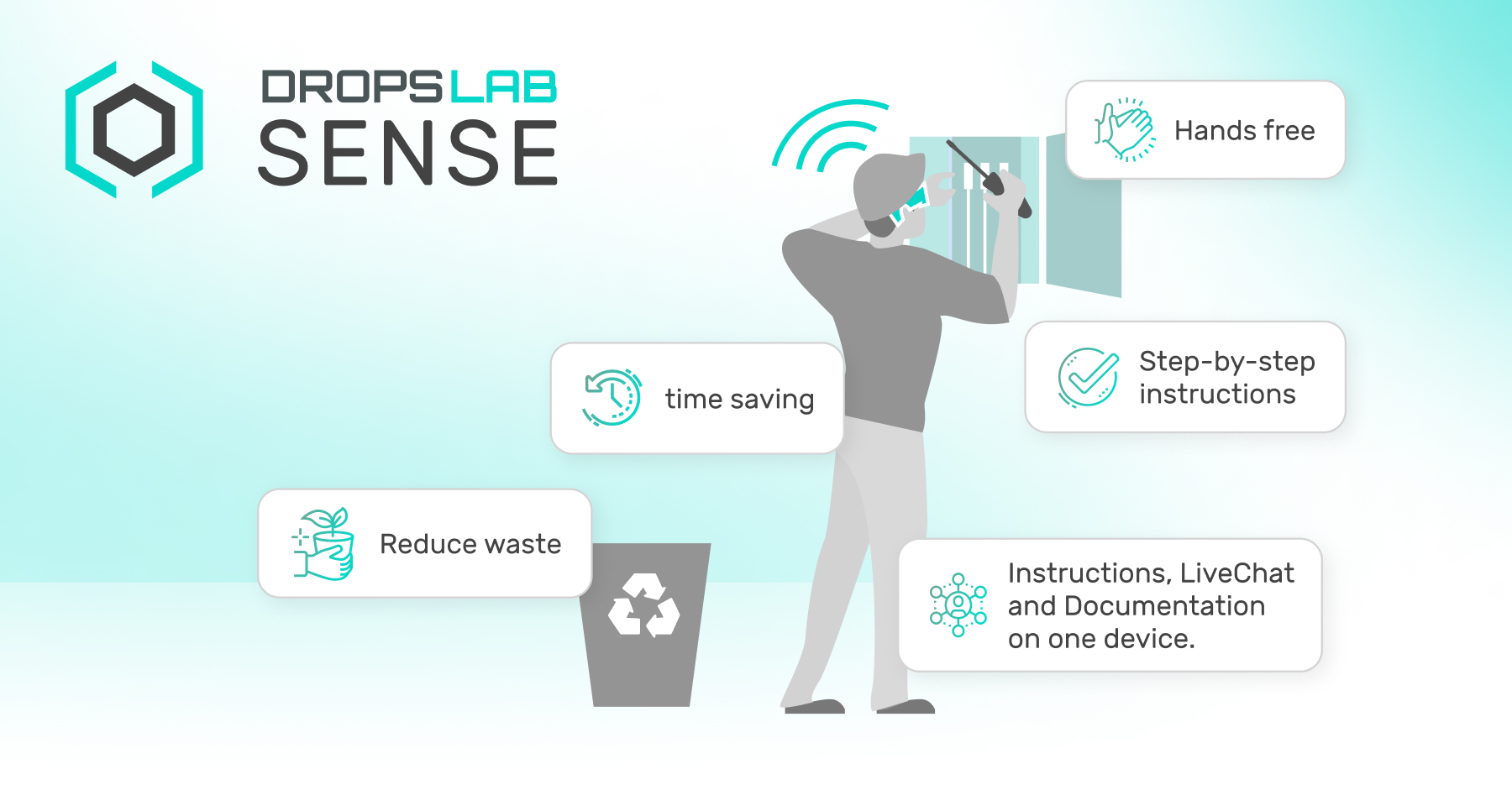
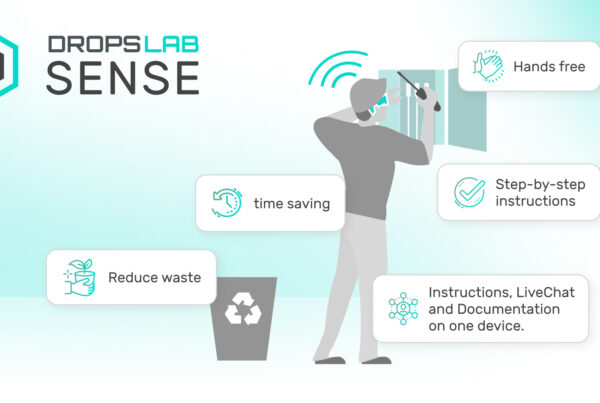
Simplifying Data Collection with Dropslab
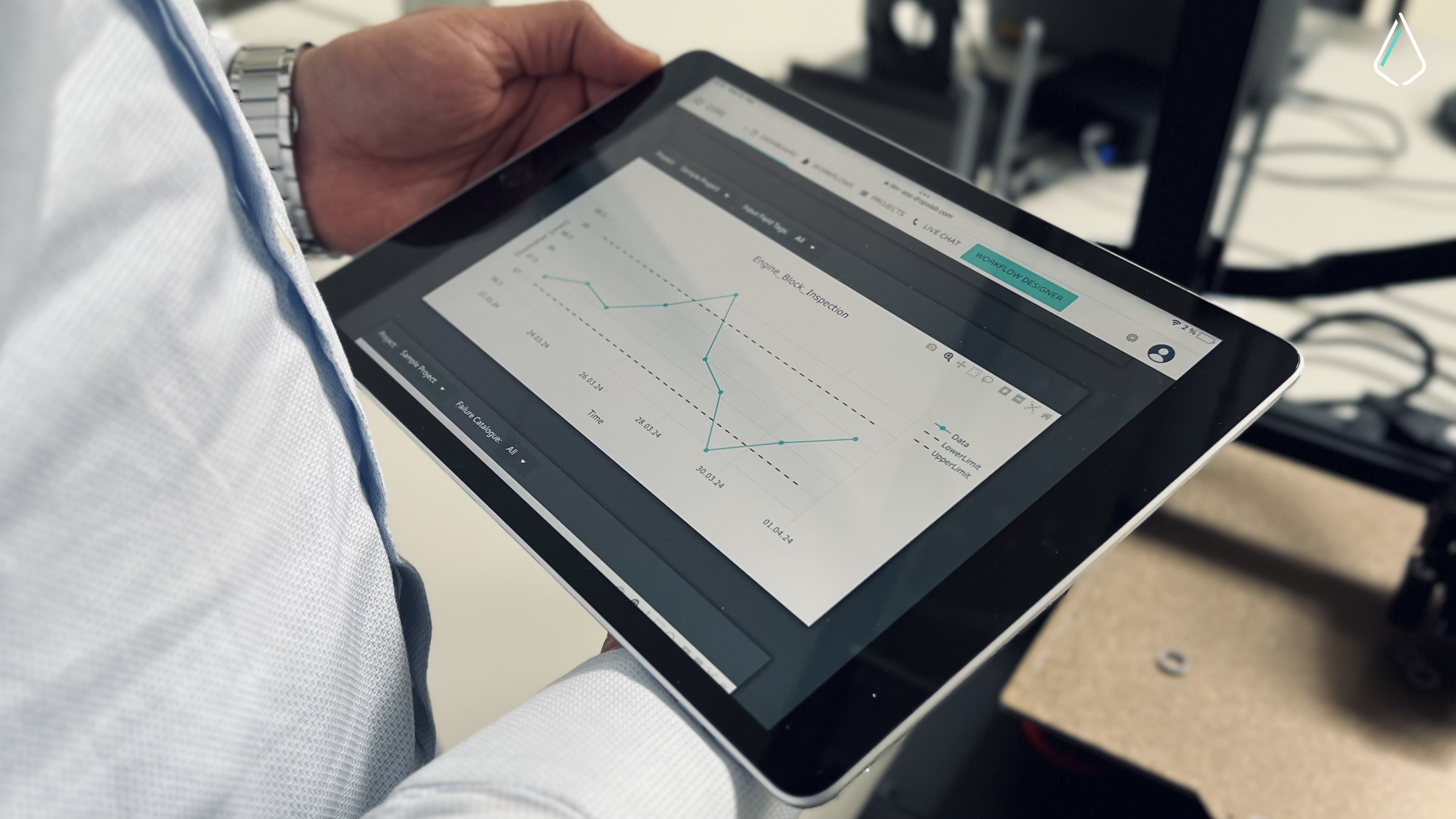
Following ALCOA+ principles are important and still challenging for manufacturers depending on paper-based work instructions and records as they are prone to errors and incomplete insights. To solve this, manufacturers and organisations are shifting to solutions like Dropslab Sense to digitise their workflows and eliminate human error.
Our solutions allow manufacturers to track every activity, product, and individual involved in the production line, producing a digital audit path that makes sure compliance with ALCOA+ principles.
Interested in learning more about how Dropslab Technologies can help simplify your production processes and records?
OR
FAQs
1. What is the ALCOA framework for data integrity in life sciences manufacturing?
The ALCOA framework is a set of principles developed by the FDA to ensure data integrity in life sciences manufacturing. It stands for Attributable, Legible, Contemporaneous, Original, and Accurate. These principles help manufacturers ensure that their data is properly collected, recorded, and maintained throughout the production process to comply with regulatory requirements.
2. What are the additional principles included in ALCOA+?
Apart from the original ALCOA principles, ALCOA+ includes four more principles to enhance data integrity: Complete, Consistent, Enduring, and Available. These principles ensure that data is comprehensive, reliable, and accessible for the long term, supporting the traceability and accuracy needed for regulatory compliance.
3. What are the main benefits of integrating remote assistance into the production line?
Data integrity is essential in life sciences manufacturing because it ensures that all data collected during the production process is accurate, traceable, and compliant with regulatory standards. This helps manufacturers maintain product safety, meet Good Manufacturing Practice (GMP) standards, and pass periodic audits, ultimately safeguarding consumer well-being and improving business decision-making.
4. How can manufacturers ensure data integrity using digital solutions?
Manufacturers can ensure data integrity by digitising their processes. Solutions like Dropslab Sense, developed by Droplsab, allow manufacturers to move away from paper-based systems, minimising errors and ensuring accurate, legible, and timely records. These digital tools create a comprehensive audit trail and help manufacturers follow ALCOA+ principles while improving efficiency and compliance.
5. How does Dropslab help manufacturers comply with ALCOA+ principles?
Dropslab offers digital solutions that help life sciences manufacturers track every activity, product, and individual involved in the production process. By automating data collection and creating a digital audit trail, Dropslab ensures that data is attributable, accurate, legible, and meets all ALCOA+ principles, thereby simplifying compliance and improving operational efficiency.